While we try to keep things accurate, this content is part of an ongoing experiment and may not always be reliable.
Please double-check important details — we’re not responsible for how the information is used.
Cell Biology
Unlocking the Secrets of Deadly Tropical Diseases: A New Target for Treatment
The efforts of a research team give hope for new treatment approaches for dangerous tropical diseases. The researchers have compiled a high-precision inventory of the membrane proteins of cell organelles of the African sleeping sickness pathogen.
Behavioral Science
The Sugar that Sparked Life: Unraveling the Mystery of Ribose’s Preeminence in RNA Development
What made ribose the sugar of choice for life’s code? Scientists at Scripps Research may have cracked a major part of this mystery. Their experiments show that ribose binds more readily and selectively to phosphate compared to other similar sugars, forming a structure ideal for RNA formation. This discovery hints at how nature might have selected specific molecules long before enzymes or life existed, and could reshape our understanding of life’s chemical origins.
Cell Biology
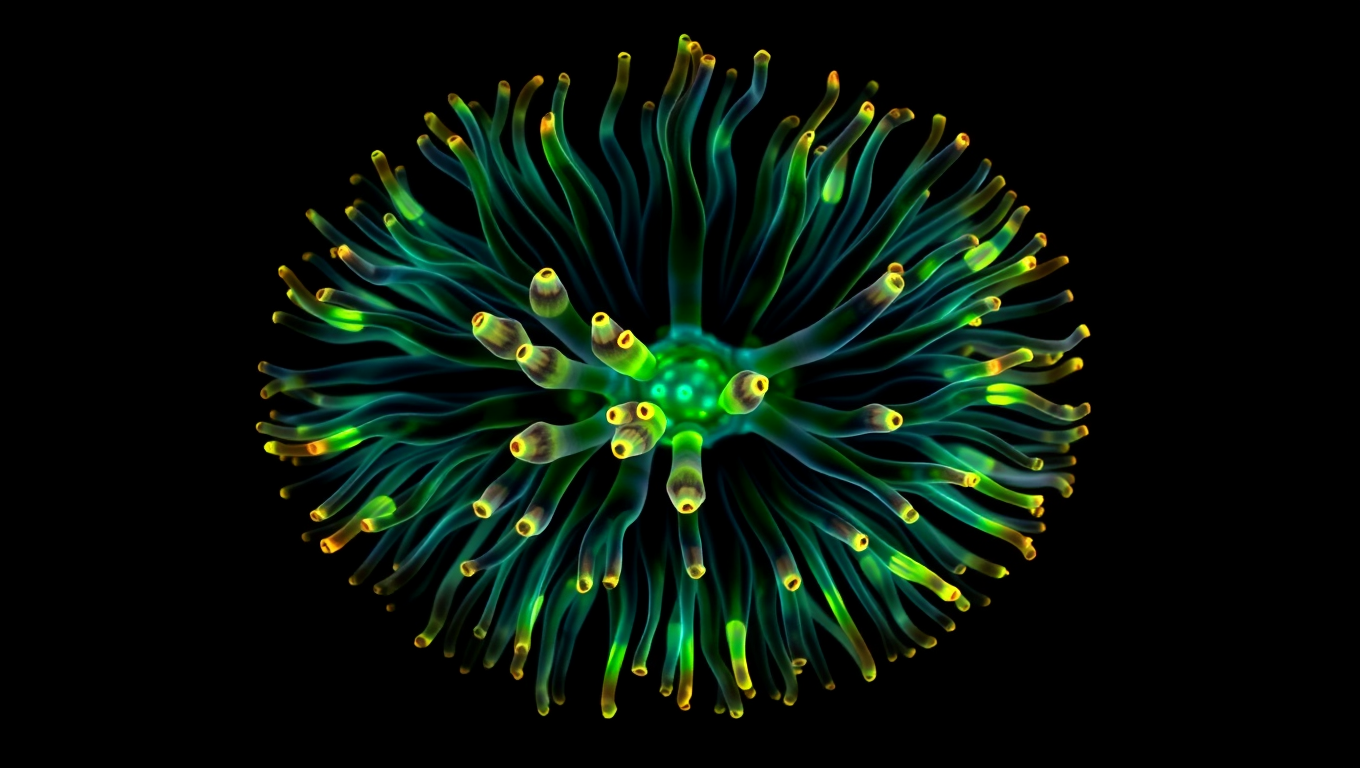
A 600-Million-Year-Old Body Blueprint Uncovered in Sea Anemones
Sea anemones may hold the key to the ancient origins of body symmetry. A study from the University of Vienna shows they use a molecular mechanism known as BMP shuttling, once thought unique to bilaterally symmetrical animals like humans, insects, and worms. This surprising discovery implies that the blueprint for forming a back-to-belly body axis could date back over 600 million years, to a common ancestor of cnidarians and bilaterians.
Biology
Unraveling Microtubule Mysteries: Scientists Crack Code on Cellular Scaffolding Secrets
Scientists found out how naturally unstable filaments decide whether to grow or to shorten.
-

 Detectors11 months ago
Detectors11 months agoA New Horizon for Vision: How Gold Nanoparticles May Restore People’s Sight
-

 Earth & Climate12 months ago
Earth & Climate12 months agoRetiring Abroad Can Be Lonely Business
-

 Cancer11 months ago
Cancer11 months agoRevolutionizing Quantum Communication: Direct Connections Between Multiple Processors
-

 Albert Einstein12 months ago
Albert Einstein12 months agoHarnessing Water Waves: A Breakthrough in Controlling Floating Objects
-

 Chemistry11 months ago
Chemistry11 months ago“Unveiling Hidden Patterns: A New Twist on Interference Phenomena”
-

 Earth & Climate11 months ago
Earth & Climate11 months agoHousehold Electricity Three Times More Expensive Than Upcoming ‘Eco-Friendly’ Aviation E-Fuels, Study Reveals
-

 Agriculture and Food11 months ago
Agriculture and Food11 months ago“A Sustainable Solution: Researchers Create Hybrid Cheese with 25% Pea Protein”
-

 Diseases and Conditions12 months ago
Diseases and Conditions12 months agoReducing Falls Among Elderly Women with Polypharmacy through Exercise Intervention