While we try to keep things accurate, this content is part of an ongoing experiment and may not always be reliable.
Please double-check important details — we’re not responsible for how the information is used.
Behavioral Science
Echidna Pseudo-Pouch Microbiome Shifts During Lactation Helps Young Thrive
Research shows microbial communities in echidna pseudo-pouches undergo dramatic changes while the animal is lactating, which could help in creating an environment for their young, known as puggles, to thrive.

Behavioral Science
“Rewired for Romance: Scientists Give Gift-Giving Behavior to Singing Fruit Flies”
By flipping a single genetic switch, researchers made one fruit fly species adopt the gift-giving courtship of another, showing how tiny brain rewiring can drive evolutionary change.
Behavioral Science
The Amazing Ant Strategy That Can Revolutionize Robotics
Weaver ants have cracked a teamwork puzzle that humans have struggled with for over a century — instead of slacking off as their group grows, they work harder. These tiny architects not only build elaborate leaf nests but also double their pulling power when more ants join in. Using a “force ratchet” system where some pull while others anchor, they outperform the efficiency of human teams and could inspire revolutionary advances in robotics cooperation.
Alternative Medicine
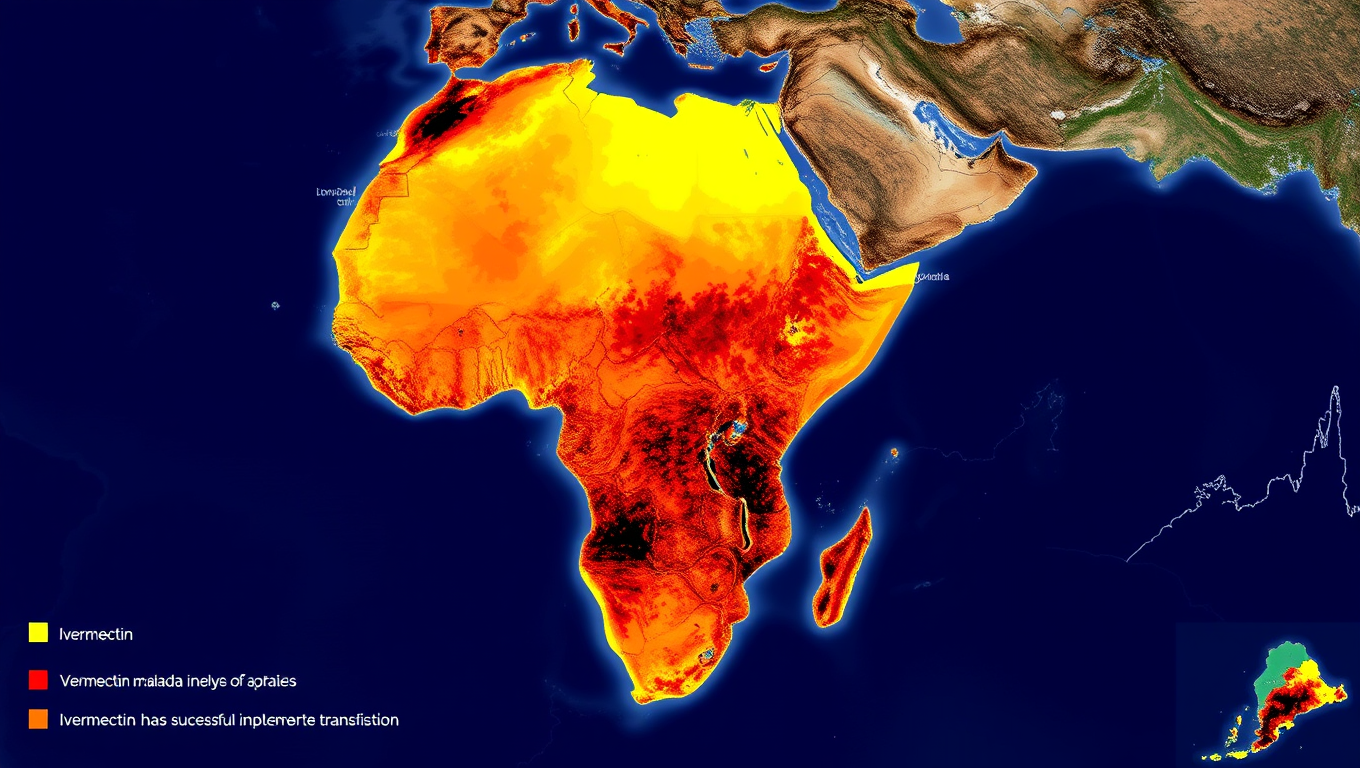
Ivermectin: The Mosquito-Killing Pill That Dropped Malaria by 26%
A groundbreaking study has revealed that the mass administration of ivermectin—a drug once known for treating river blindness and scabies—can significantly reduce malaria transmission when used in conjunction with bed nets.
-

 Detectors11 months ago
Detectors11 months agoA New Horizon for Vision: How Gold Nanoparticles May Restore People’s Sight
-

 Cancer12 months ago
Cancer12 months agoRevolutionizing Quantum Communication: Direct Connections Between Multiple Processors
-

 Earth & Climate12 months ago
Earth & Climate12 months agoRetiring Abroad Can Be Lonely Business
-

 Albert Einstein12 months ago
Albert Einstein12 months agoHarnessing Water Waves: A Breakthrough in Controlling Floating Objects
-

 Chemistry11 months ago
Chemistry11 months ago“Unveiling Hidden Patterns: A New Twist on Interference Phenomena”
-

 Earth & Climate11 months ago
Earth & Climate11 months agoHousehold Electricity Three Times More Expensive Than Upcoming ‘Eco-Friendly’ Aviation E-Fuels, Study Reveals
-

 Agriculture and Food12 months ago
Agriculture and Food12 months ago“A Sustainable Solution: Researchers Create Hybrid Cheese with 25% Pea Protein”
-

 Diseases and Conditions12 months ago
Diseases and Conditions12 months agoReducing Falls Among Elderly Women with Polypharmacy through Exercise Intervention