While we try to keep things accurate, this content is part of an ongoing experiment and may not always be reliable.
Please double-check important details — we’re not responsible for how the information is used.
Biochemistry
Revolutionizing Gene Delivery with ENVLPE: A Breakthrough in Precision Medicine
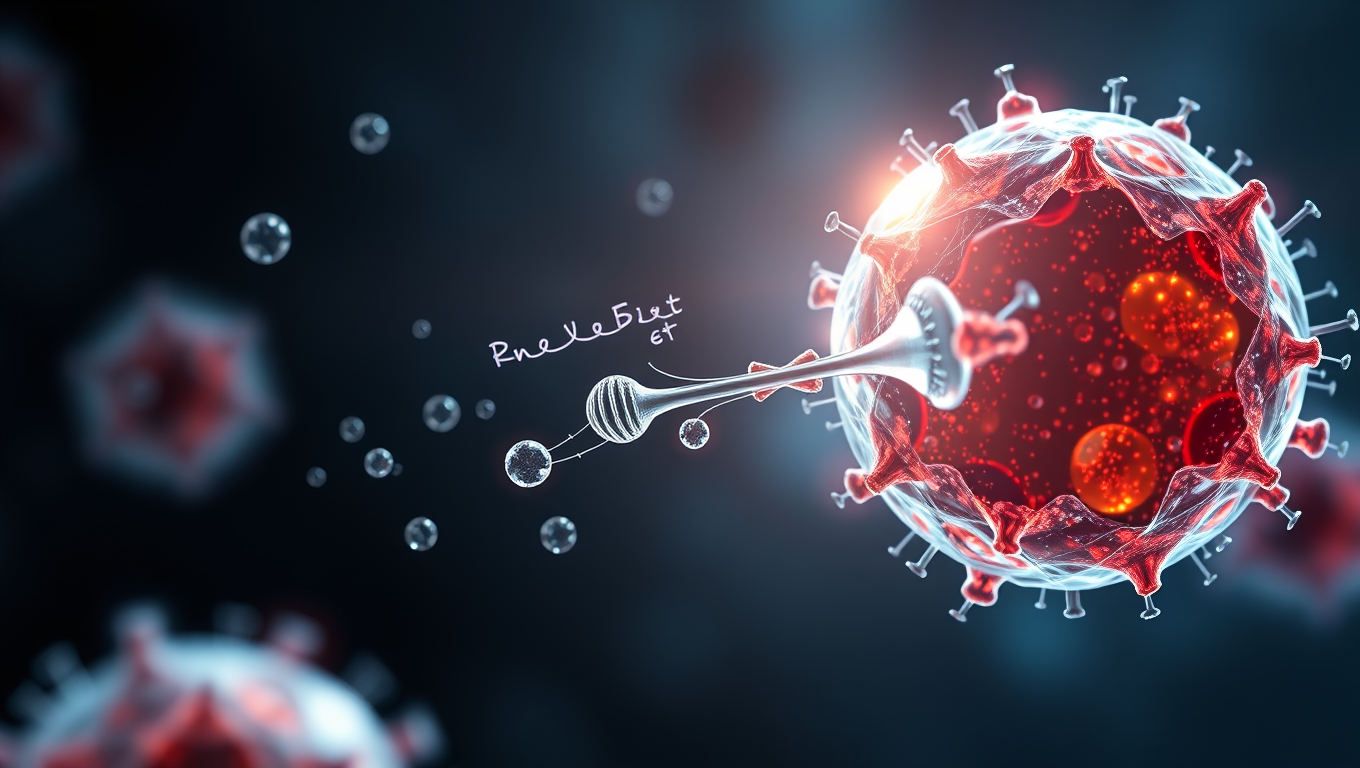
A research team has developed an advanced delivery system that transports gene-editing tools based on the CRISPR/Cas9 gene-editing system into living cells with significantly greater efficiency than before. Their technology, ENVLPE, uses engineered non-infectious virus-like particles to precisely correct defective genes — demonstrated successfully in living mouse models that are blind due to a mutation. This system also holds promise for advancing cancer therapy by enabling precise genetic manipulation of engineered immune cells making them more universally compatible and thus more accessible for a larger group of cancer patients.
Biochemistry
Shape-Shifting Catalysts: Revolutionizing Green Chemistry with a Single Atom
A team in Milan has developed a first-of-its-kind single-atom catalyst that acts like a molecular switch, enabling cleaner, more adaptable chemical reactions. Stable, recyclable, and eco-friendly, it marks a major step toward programmable sustainable chemistry.
Biochemistry
Scientists Finally Tame the Impossible: A Stable 48-Atom Carbon Ring is Achieved
Researchers have synthesized a stable cyclo[48]carbon, a unique 48-carbon ring that can be studied in solution at room temperature, a feat never achieved before.
Biochemistry
“Revolutionizing Medicine: A 100x Faster Path to Life-Saving Drugs with Metal Carbenes”
Using a clever combo of iron and radical chemistry, scientists have unlocked a safer, faster way to create carbenes molecular powerhouses key to modern medicine and materials. It s 100x more efficient than previous methods.
-

 Detectors11 months ago
Detectors11 months agoA New Horizon for Vision: How Gold Nanoparticles May Restore People’s Sight
-

 Cancer12 months ago
Cancer12 months agoRevolutionizing Quantum Communication: Direct Connections Between Multiple Processors
-

 Earth & Climate12 months ago
Earth & Climate12 months agoRetiring Abroad Can Be Lonely Business
-

 Albert Einstein12 months ago
Albert Einstein12 months agoHarnessing Water Waves: A Breakthrough in Controlling Floating Objects
-

 Chemistry11 months ago
Chemistry11 months ago“Unveiling Hidden Patterns: A New Twist on Interference Phenomena”
-

 Earth & Climate12 months ago
Earth & Climate12 months agoHousehold Electricity Three Times More Expensive Than Upcoming ‘Eco-Friendly’ Aviation E-Fuels, Study Reveals
-

 Agriculture and Food12 months ago
Agriculture and Food12 months ago“A Sustainable Solution: Researchers Create Hybrid Cheese with 25% Pea Protein”
-

 Diseases and Conditions12 months ago
Diseases and Conditions12 months agoReducing Falls Among Elderly Women with Polypharmacy through Exercise Intervention