While we try to keep things accurate, this content is part of an ongoing experiment and may not always be reliable.
Please double-check important details — we’re not responsible for how the information is used.
Batteries
Harnessing Nuclear Power: A Revolutionary Battery that Could Last a Lifetime
Lithium-ion batteries, used in consumer devices and electric vehicles, typically last hours or days between charges. However, with repeated use, they degrade and need to be charged more frequently. Now, researchers are considering radiocarbon as a source for safe, small and affordable nuclear batteries that could last decades or longer without charging.

Batteries
“Reviving ‘Dead’ Batteries: The Path to a Greener Future”
Lithium battery recycling offers a powerful solution to rising demand, with discarded batteries still holding most of their valuable materials. Compared to mining, recycling slashes emissions and resource use while unlocking major economic potential. Yet infrastructure, policy, and technology hurdles must still be overcome.
Batteries
“Revolutionizing Energy Storage: AI-Driven Discovery of New Materials for Multivalent-Ion Batteries”
AI is helping scientists crack the code on next-gen batteries that could replace lithium-ion tech. By discovering novel porous materials, researchers may have paved the way for more powerful and sustainable energy storage using abundant elements like magnesium.
Batteries
Unlocking Battery Secrets at the Atomic Scale
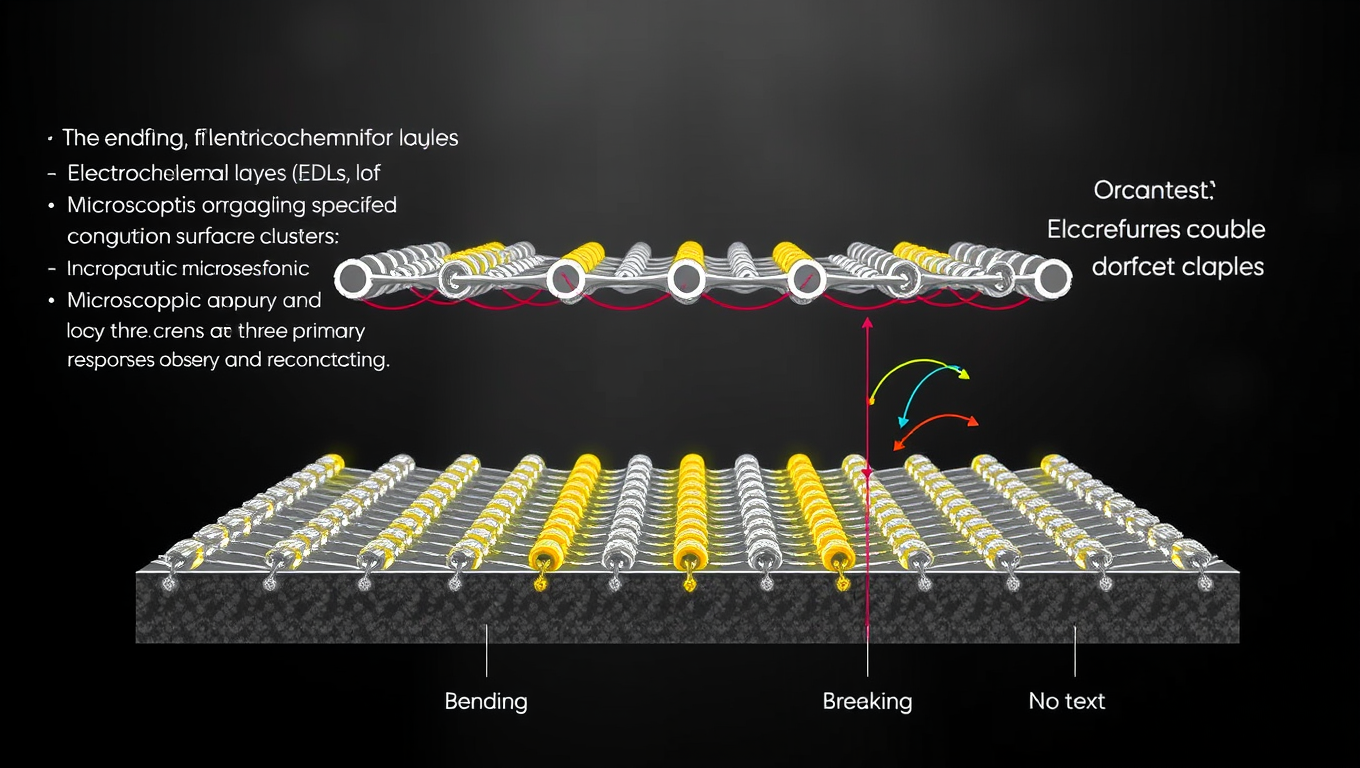
Scientists have cracked open a mysterious layer inside batteries, using cutting-edge 3D atomic force microscopy to capture the dynamic molecular structures at their solid-liquid interfaces. These once-invisible electrical double layers (EDLs) twist, break, and reform in response to surface irregularities phenomena never seen before in real-world battery systems. The findings don t just refine our understanding of how batteries work at the microscopic level they could fundamentally change how we build and design next-generation energy storage.
-

 Detectors9 months ago
Detectors9 months agoA New Horizon for Vision: How Gold Nanoparticles May Restore People’s Sight
-

 Earth & Climate10 months ago
Earth & Climate10 months agoRetiring Abroad Can Be Lonely Business
-

 Cancer10 months ago
Cancer10 months agoRevolutionizing Quantum Communication: Direct Connections Between Multiple Processors
-

 Albert Einstein10 months ago
Albert Einstein10 months agoHarnessing Water Waves: A Breakthrough in Controlling Floating Objects
-

 Chemistry10 months ago
Chemistry10 months ago“Unveiling Hidden Patterns: A New Twist on Interference Phenomena”
-

 Earth & Climate10 months ago
Earth & Climate10 months agoHousehold Electricity Three Times More Expensive Than Upcoming ‘Eco-Friendly’ Aviation E-Fuels, Study Reveals
-

 Agriculture and Food10 months ago
Agriculture and Food10 months ago“A Sustainable Solution: Researchers Create Hybrid Cheese with 25% Pea Protein”
-

 Diseases and Conditions10 months ago
Diseases and Conditions10 months agoReducing Falls Among Elderly Women with Polypharmacy through Exercise Intervention