While we try to keep things accurate, this content is part of an ongoing experiment and may not always be reliable.
Please double-check important details — we’re not responsible for how the information is used.
Biochemistry Research
A Game-Changing Device for TB Diagnoses: Revolutionizing Healthcare for Children
This handheld device is the first that can detect tuberculosis in saliva, in addition to blood and sputum samples, an important breakthrough for testing children and HIV patients, who struggle to produce sputum. The device was found to deliver rapid, accurate results in under an hour, offering a cost-effective and accessible solution for diagnosing TB in resource-limited areas.
Biochemistry Research
The Double Edge of Love and War: How Female Earwigs Evolved Deadly Claws for Mate Competition
Female earwigs may be evolving exaggerated weaponry just like males. A study from Toho University found that female forceps, once assumed to be passive tools, show the same kind of outsized growth linked to sexual selection as the male’s iconic pincers. This means that female earwigs might be fighting for mates too specifically for access to non-aggressive males challenging long-standing assumptions in evolutionary biology.
Biochemistry Research
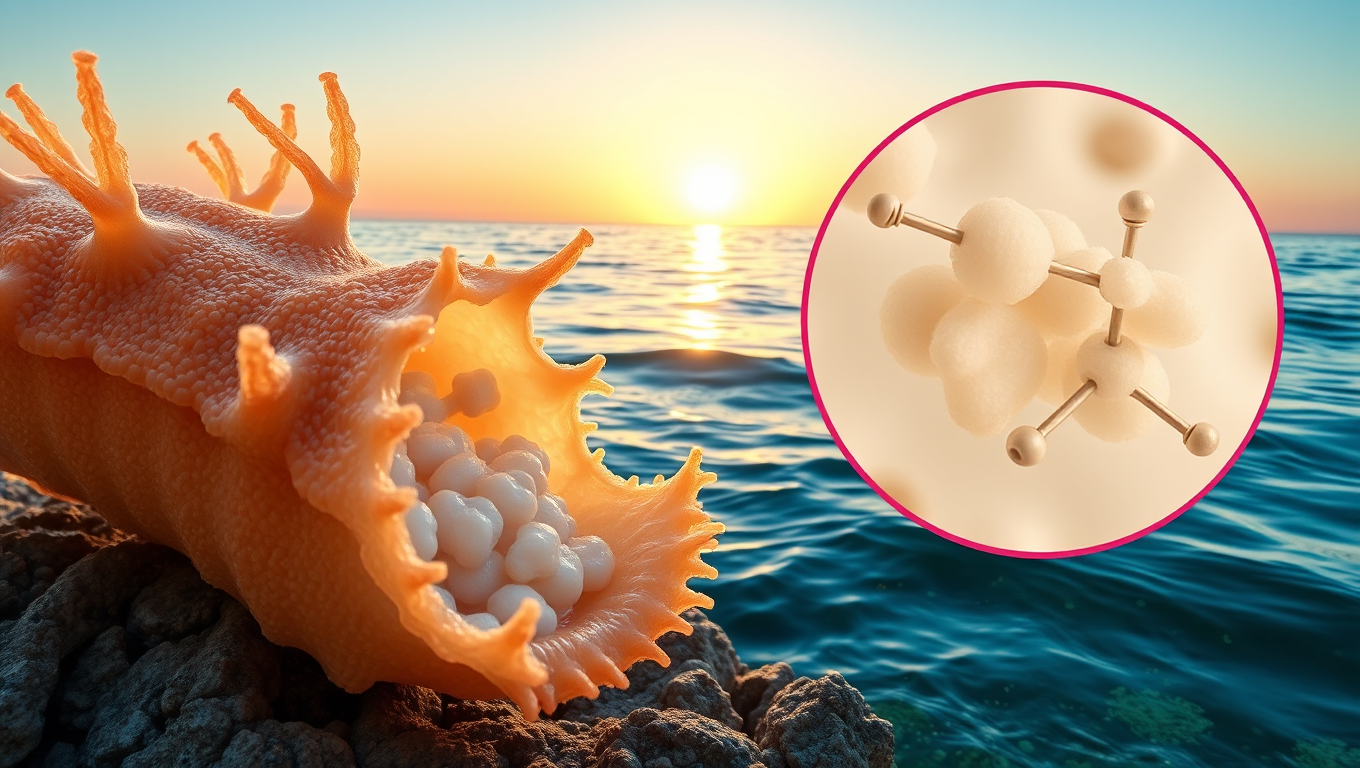
Unlocking Nature’s Secrets: Scientists Discover Natural Cancer-Fighting Sugar in Sea Cucumbers
Sea cucumbers, long known for cleaning the ocean floor, may also harbor a powerful cancer-fighting secret. Scientists discovered a unique sugar in these marine creatures that can block Sulf-2, an enzyme that cancer cells use to spread. Unlike traditional medications, this compound doesn t cause dangerous blood clotting issues and offers a cleaner, potentially more sustainable way to develop carbohydrate-based drugs if scientists can find a way to synthesize it in the lab.
Air Quality
Unlocking the Secrets of Environmental DNA: A Powerful Tool for Wildlife and Human Surveillance
Environmental DNA from the air, captured with simple air filters, can track everything from illegal drugs to the wildlife it was originally designed to study.
-

 Detectors2 months ago
Detectors2 months agoA New Horizon for Vision: How Gold Nanoparticles May Restore People’s Sight
-

 Earth & Climate4 months ago
Earth & Climate4 months agoRetiring Abroad Can Be Lonely Business
-

 Cancer3 months ago
Cancer3 months agoRevolutionizing Quantum Communication: Direct Connections Between Multiple Processors
-

 Agriculture and Food3 months ago
Agriculture and Food3 months ago“A Sustainable Solution: Researchers Create Hybrid Cheese with 25% Pea Protein”
-

 Diseases and Conditions4 months ago
Diseases and Conditions4 months agoReducing Falls Among Elderly Women with Polypharmacy through Exercise Intervention
-

 Earth & Climate3 months ago
Earth & Climate3 months agoHousehold Electricity Three Times More Expensive Than Upcoming ‘Eco-Friendly’ Aviation E-Fuels, Study Reveals
-

 Chemistry3 months ago
Chemistry3 months ago“Unveiling Hidden Patterns: A New Twist on Interference Phenomena”
-

 Albert Einstein4 months ago
Albert Einstein4 months agoHarnessing Water Waves: A Breakthrough in Controlling Floating Objects