While we try to keep things accurate, this content is part of an ongoing experiment and may not always be reliable.
Please double-check important details — we’re not responsible for how the information is used.
Behavior
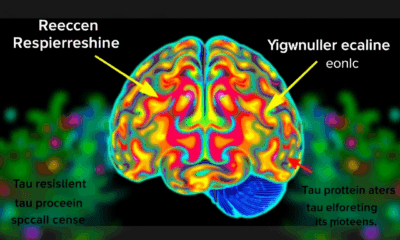
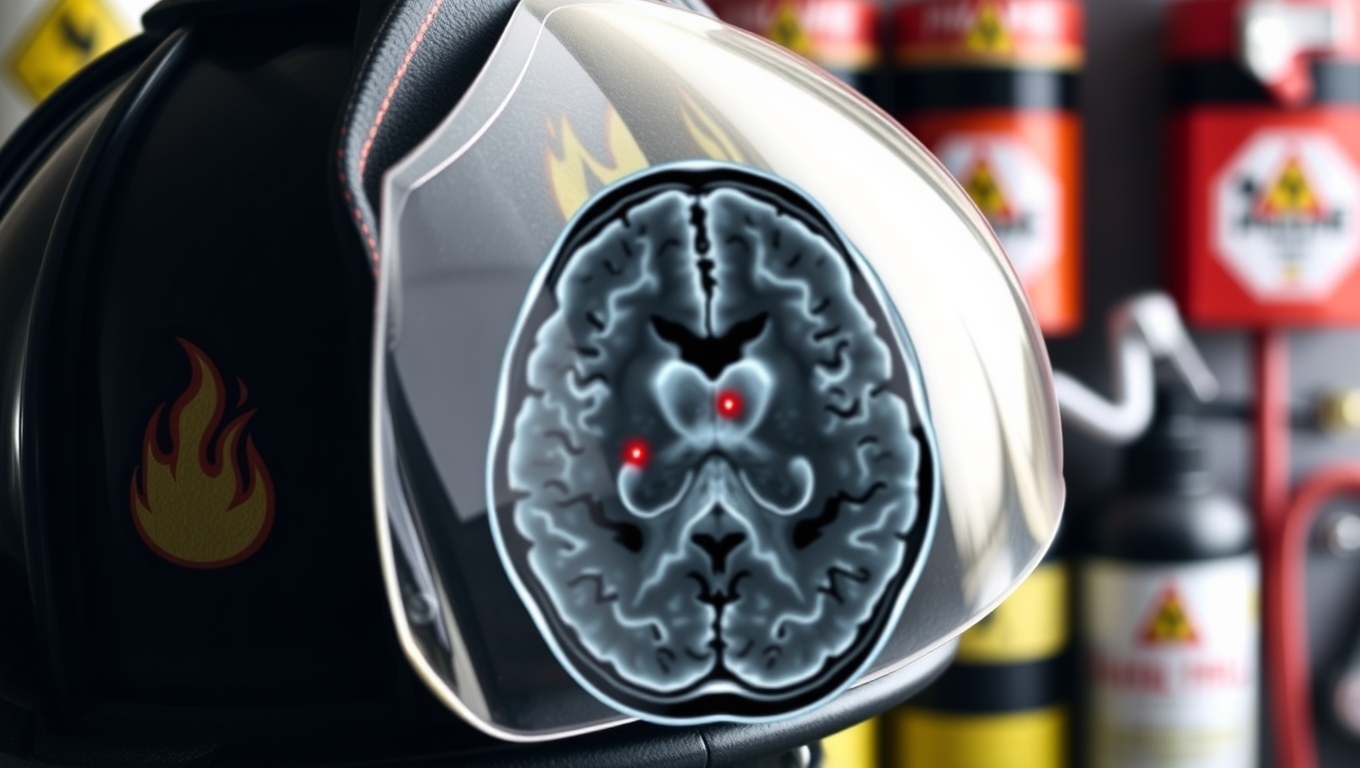
Firefighters’ Genetic Mutations Linked to Toxin Exposure in Brain Tumors
In a study comparing the glioma tumors of firefighters and non-fighters, researchers found a mutational signature tied to exposure to haloalkanes, which are used in flame retardants, fire extinguishers, and pesticides.
Behavior
Unraveling the Mind: How a Scent Can Change Your Decisions
Mice taught to link smells with tastes, and later fear, revealed how the amygdala teams up with cortical regions to let the brain draw powerful indirect connections. Disabling this circuit erased the links, hinting that similar pathways in humans could underlie disorders like PTSD and psychosis, and might be tuned with future brain-modulation therapies.
Autism
Unpacking the Gene That Hijacks Fear: How PTEN Rewires the Brain’s Anxiety Circuit
Deleting a gene called PTEN in certain brain cells disrupts the brain’s fear circuitry and triggers anxiety-like behavior in mice — key traits seen in autism. Researchers mapped how this genetic tweak throws off the brain’s delicate balance of excitation and inhibition in the amygdala, offering deep insights into how one gene can drive specific ASD symptoms.
Amyotrophic Lateral Sclerosis
“Reviving Memories: Gene Therapy Shows Promise in Reversing Alzheimer’s Disease in Mice”
UC San Diego scientists have created a gene therapy that goes beyond masking Alzheimer’s symptoms—it may actually restore brain function. In mice, the treatment protected memory and altered diseased brain cells to behave more like healthy ones.
-

 Detectors3 months ago
Detectors3 months agoA New Horizon for Vision: How Gold Nanoparticles May Restore People’s Sight
-

 Earth & Climate4 months ago
Earth & Climate4 months agoRetiring Abroad Can Be Lonely Business
-

 Cancer4 months ago
Cancer4 months agoRevolutionizing Quantum Communication: Direct Connections Between Multiple Processors
-

 Agriculture and Food4 months ago
Agriculture and Food4 months ago“A Sustainable Solution: Researchers Create Hybrid Cheese with 25% Pea Protein”
-

 Diseases and Conditions4 months ago
Diseases and Conditions4 months agoReducing Falls Among Elderly Women with Polypharmacy through Exercise Intervention
-

 Chemistry3 months ago
Chemistry3 months ago“Unveiling Hidden Patterns: A New Twist on Interference Phenomena”
-

 Albert Einstein4 months ago
Albert Einstein4 months agoHarnessing Water Waves: A Breakthrough in Controlling Floating Objects
-

 Earth & Climate4 months ago
Earth & Climate4 months agoHousehold Electricity Three Times More Expensive Than Upcoming ‘Eco-Friendly’ Aviation E-Fuels, Study Reveals