While we try to keep things accurate, this content is part of an ongoing experiment and may not always be reliable.
Please double-check important details — we’re not responsible for how the information is used.
Alzheimer's Research
Microplastics in Medical Infusion Bags: A Hidden Health Risk
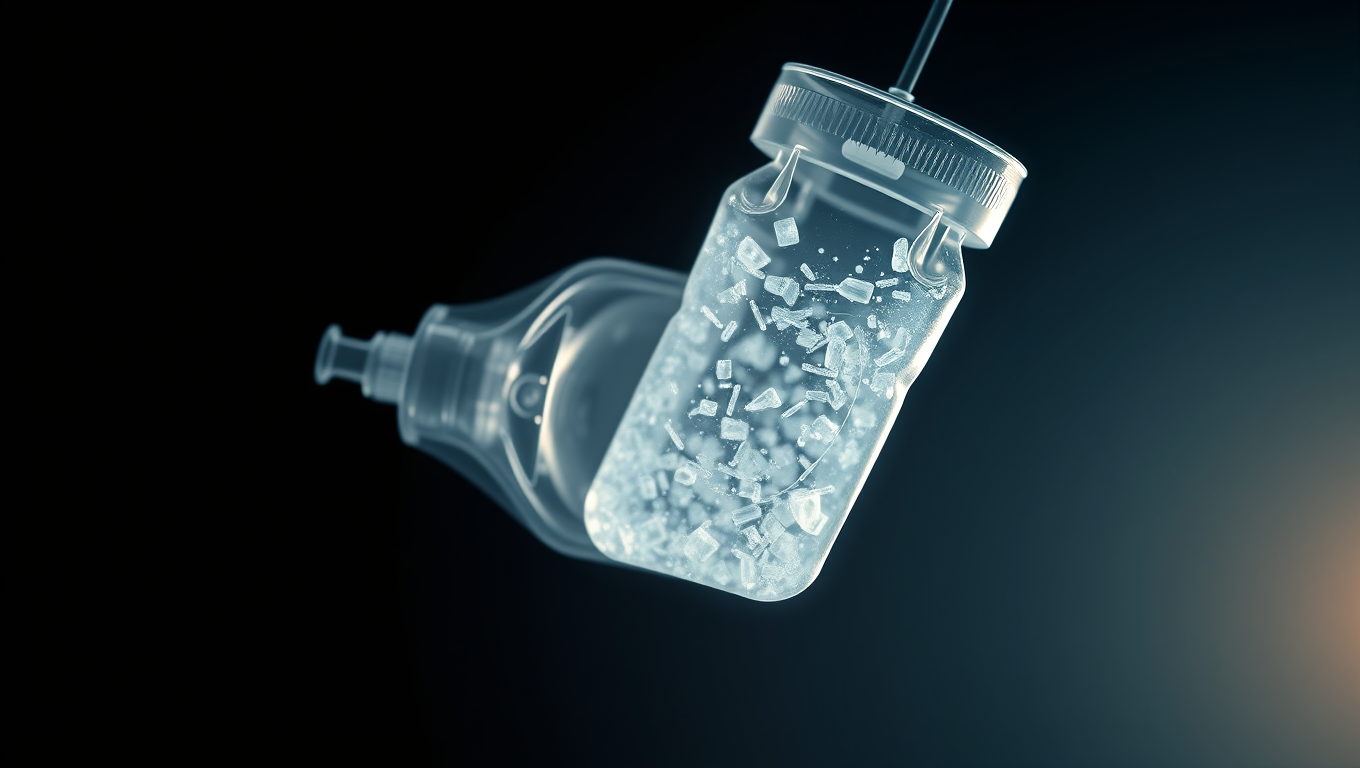
Microplastics have been found almost everywhere that scientists have looked for them. Now these bits of plastic — from 1 to 62 micrometers long — have been found in the filtered solutions used for medical intravenous (IV) infusions. The researchers estimate that thousands of plastic particles could be delivered directly to a person’s bloodstream from a single 8.4-ounce (250-milliliter) bag of infusion fluid.
Alzheimer's
Uncovering the Hidden Culprits Behind Alzheimer’s Disease
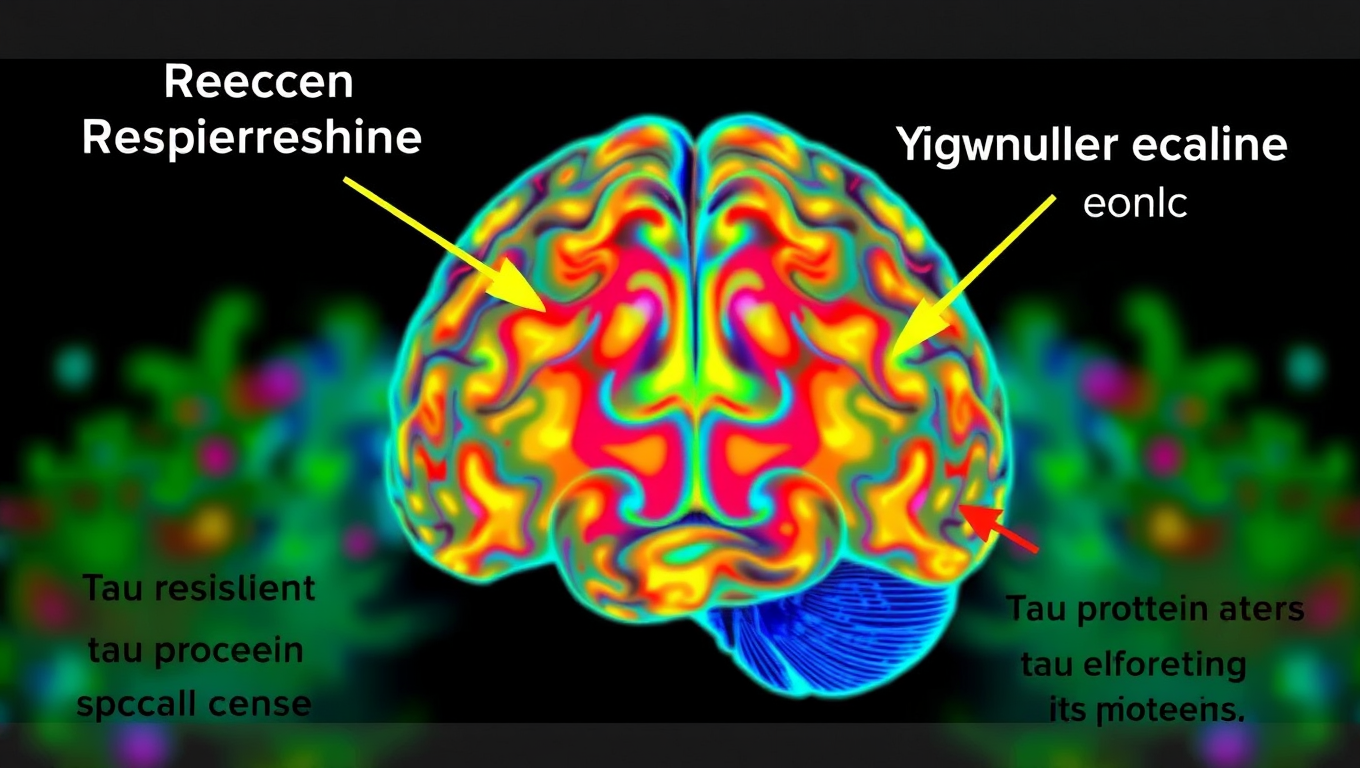
A surprising new study has uncovered over 200 misfolded proteins in the brains of aging rats with cognitive decline, beyond the infamous amyloid and tau plaques long blamed for Alzheimer’s. These shape-shifting proteins don’t clump into visible plaques, making them harder to detect but potentially just as harmful. Scientists believe these “stealth” molecules could evade the brain’s cleanup systems and quietly impair memory and brain function. The discovery opens a new frontier in understanding dementia and could lead to entirely new targets for treatment and prevention.
Alzheimer's
Uncovering the Hidden Defenses Against Alzheimer’s Disease: A Breakthrough Study on Brain Resilience
Scientists at UCSF combined advanced brain-network modeling, genetics, and imaging to reveal how tau protein travels through neural highways and how certain genes either accelerate its toxic journey or shield brain regions from damage. Their extended Network Diffusion Model pinpoints four gene categories that govern vulnerability or resilience, reshaping our view of Alzheimer’s progression and spotlighting fresh therapeutic targets.
Alternative Medicine
A Pain-Free Patch Revolutionizes Cancer Detection with Nanoneedles
A new nanotechnology breakthrough may soon eliminate the need for painful biopsies. Scientists have developed a patch filled with nanoneedles thinner than a human hair that can painlessly extract molecular data from tissues without removing or damaging them. This enables real-time disease monitoring, particularly for conditions like brain cancer and Alzheimer s, and could radically change how doctors diagnose and track disease. The patch works quickly, integrates with common medical tools, and provides results using AI, opening doors to personalized medicine and better surgical decisions.
-

 Detectors3 months ago
Detectors3 months agoA New Horizon for Vision: How Gold Nanoparticles May Restore People’s Sight
-

 Earth & Climate4 months ago
Earth & Climate4 months agoRetiring Abroad Can Be Lonely Business
-

 Cancer4 months ago
Cancer4 months agoRevolutionizing Quantum Communication: Direct Connections Between Multiple Processors
-

 Agriculture and Food4 months ago
Agriculture and Food4 months ago“A Sustainable Solution: Researchers Create Hybrid Cheese with 25% Pea Protein”
-

 Diseases and Conditions4 months ago
Diseases and Conditions4 months agoReducing Falls Among Elderly Women with Polypharmacy through Exercise Intervention
-

 Albert Einstein4 months ago
Albert Einstein4 months agoHarnessing Water Waves: A Breakthrough in Controlling Floating Objects
-

 Chemistry4 months ago
Chemistry4 months ago“Unveiling Hidden Patterns: A New Twist on Interference Phenomena”
-

 Earth & Climate4 months ago
Earth & Climate4 months agoHousehold Electricity Three Times More Expensive Than Upcoming ‘Eco-Friendly’ Aviation E-Fuels, Study Reveals