While we try to keep things accurate, this content is part of an ongoing experiment and may not always be reliable.
Please double-check important details — we’re not responsible for how the information is used.
Dark Matter
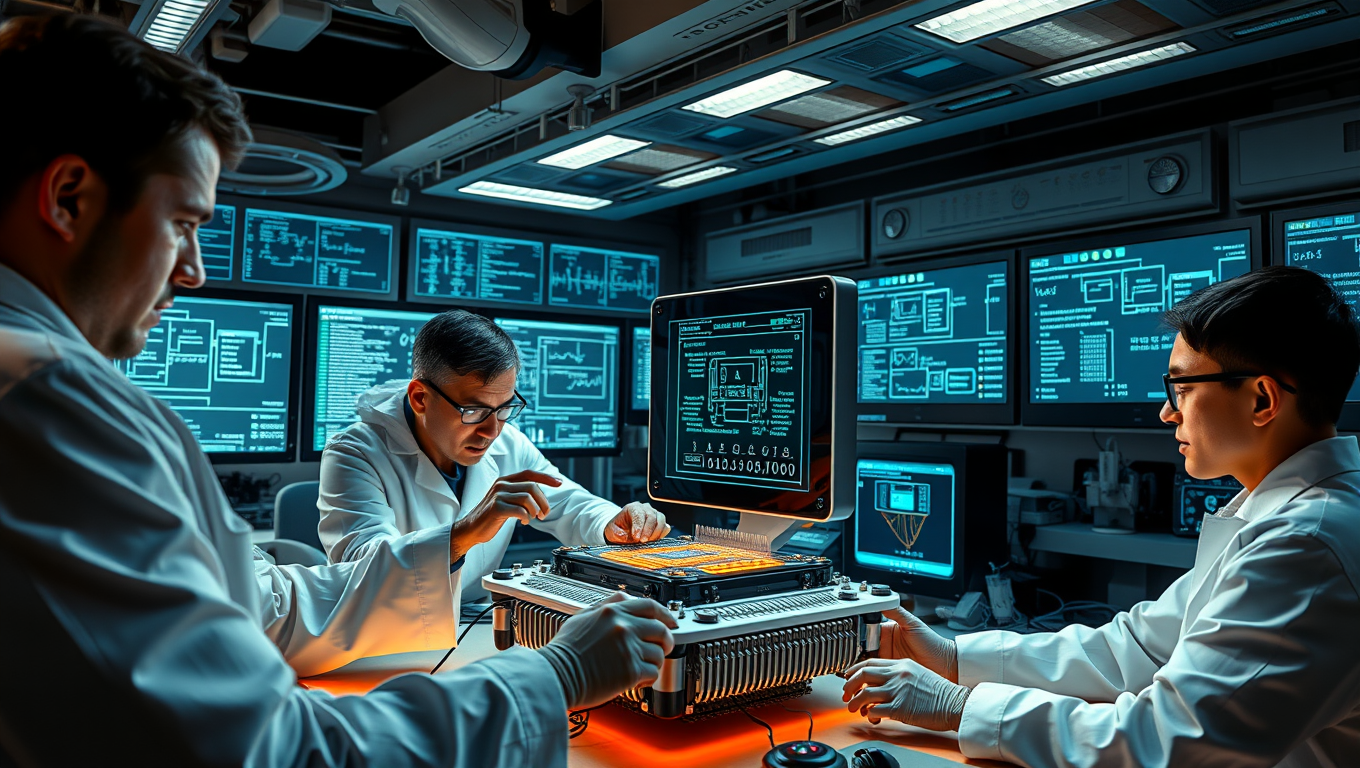
Unlocking the Secrets of the Universe: A Breakthrough in Quantum Technology
A groundbreaking quantum device small enough to fit in your hand could one day answer one of the biggest questions in science — whether the multiverse is real. This tiny chip can generate extreme electromagnetic fields once only possible in massive, miles-long particle colliders. Beyond probing the fabric of reality, it could lead to powerful gamma ray lasers capable of destroying cancer cells at the atomic level, offering a glimpse into a future where the deepest mysteries of the universe and life-saving medical breakthroughs are unlocked by technology no bigger than your thumb.
Dark Matter
Clearest Mars Images Yet Reveal Stunning Terrain and Mysterious Rock Formation
Captured at a location called “Falbreen,” this 360-degree view mosaic was stitched together 96 images that were acquired May 26, 2025. In the upper image, the enhanced-color mosaic features deceptively blue skies and the 43rd rock abrasion (the white patch at center-left) of the NASA Perseverance rover’s mission at Mars. Below, in the natural-color version of the “Falbreen” panorama, colors have not been enhanced and the sky appears more reddish. Credit: NASA/JPL-Caltech/ASU/MSSS
Agriculture and Food
Unearthing Life’s Secrets: Deep Microbes Thrive without Sunlight
Chinese scientists uncovered a powerful energy source for deep Earth microbes: hydrogen and oxidants generated by rock fracturing during earthquakes. The process may also suggest how life could exist on other planets without sunlight.
Astronomy
A Star That Defied Death: The Supernova Survivor
In a spectacular image captured by the Hubble Space Telescope, the spiral galaxy NGC 1309 glows with cosmic elegance and hides a strange survivor.
-

 Detectors10 months ago
Detectors10 months agoA New Horizon for Vision: How Gold Nanoparticles May Restore People’s Sight
-

 Earth & Climate12 months ago
Earth & Climate12 months agoRetiring Abroad Can Be Lonely Business
-

 Cancer11 months ago
Cancer11 months agoRevolutionizing Quantum Communication: Direct Connections Between Multiple Processors
-

 Albert Einstein12 months ago
Albert Einstein12 months agoHarnessing Water Waves: A Breakthrough in Controlling Floating Objects
-

 Chemistry11 months ago
Chemistry11 months ago“Unveiling Hidden Patterns: A New Twist on Interference Phenomena”
-

 Earth & Climate11 months ago
Earth & Climate11 months agoHousehold Electricity Three Times More Expensive Than Upcoming ‘Eco-Friendly’ Aviation E-Fuels, Study Reveals
-

 Agriculture and Food11 months ago
Agriculture and Food11 months ago“A Sustainable Solution: Researchers Create Hybrid Cheese with 25% Pea Protein”
-

 Diseases and Conditions12 months ago
Diseases and Conditions12 months agoReducing Falls Among Elderly Women with Polypharmacy through Exercise Intervention