While we try to keep things accurate, this content is part of an ongoing experiment and may not always be reliable.
Please double-check important details — we’re not responsible for how the information is used.
COPD
A Groundbreaking Approach Offers New Hope for Inflammatory Lung Diseases
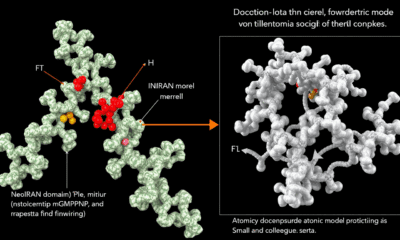
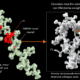
Pulmonary sarcoidosis is a lung disease characterized by granulomas — tiny clumps of immune cells that form in response to inflammation. It’s the most inflammatory of the interstitial lung diseases (ILDs), a family of conditions that all involve some level of inflammation and fibrosis, or scarring, of the lungs. In the U.S., pulmonary sarcoidosis affects around 200,000 patients. The cause is unknown, and no new treatments have been introduced in the past 70 years. Scientists now characterized a protein, HARSWHEP, that can soothe the inflammation associated with sarcoidosis by regulating white blood cells. Reducing inflammation slows the disease’s progression and results in less scarring. A phase 1b/2a clinical trial of efzofitimod, a therapeutic form of HARSWHEP, showed promising results.
COPD
Revolutionary Nanoparticles Deliver Genetic Treatments Directly to the Lungs
A scientific team has unlocked a new way to treat serious lung conditions by using specially designed nanoparticles to deliver genetic therapies straight to lung cells. This innovation could transform care for patients with cystic fibrosis or lung cancer. With a powerful combination of gene editing and RNA delivery, the system has already shown promise in animal trials. The streamlined approach not only enhances precision but also avoids harmful side effects, making it a bold leap forward in respiratory medicine.
Brain Tumor
Uncovering the Secrets of COPD: The Role of Carbon Build-up in Lung Disease
Scientists have discovered that people with COPD have lung cells that contain over three times as much soot-like carbon as those of smokers without the disease. These overloaded cells are larger and trigger more inflammation, suggesting that pollution and carbon buildup not just smoking may drive the disease.
Anemia
Hidden Risk: Three Genetic Variants That Raise Clot Risk by 180%
Genetic research in Sweden has unveiled three new gene variants that dramatically increase the risk of venous blood clots, sometimes by up to 180%. These discoveries build on existing knowledge of Factor V Leiden and suggest that genetics plays a bigger role than previously thought, especially for clots in the legs that can lead to life-threatening pulmonary embolisms.
-

 Detectors3 months ago
Detectors3 months agoA New Horizon for Vision: How Gold Nanoparticles May Restore People’s Sight
-

 Earth & Climate4 months ago
Earth & Climate4 months agoRetiring Abroad Can Be Lonely Business
-

 Cancer4 months ago
Cancer4 months agoRevolutionizing Quantum Communication: Direct Connections Between Multiple Processors
-

 Agriculture and Food4 months ago
Agriculture and Food4 months ago“A Sustainable Solution: Researchers Create Hybrid Cheese with 25% Pea Protein”
-

 Diseases and Conditions4 months ago
Diseases and Conditions4 months agoReducing Falls Among Elderly Women with Polypharmacy through Exercise Intervention
-

 Albert Einstein4 months ago
Albert Einstein4 months agoHarnessing Water Waves: A Breakthrough in Controlling Floating Objects
-

 Chemistry3 months ago
Chemistry3 months ago“Unveiling Hidden Patterns: A New Twist on Interference Phenomena”
-

 Earth & Climate3 months ago
Earth & Climate3 months agoHousehold Electricity Three Times More Expensive Than Upcoming ‘Eco-Friendly’ Aviation E-Fuels, Study Reveals