While we try to keep things accurate, this content is part of an ongoing experiment and may not always be reliable.
Please double-check important details — we’re not responsible for how the information is used.
Alternative Medicine
Brewing a Breakthrough: Scientists Turn Beer Yeast into Mini Factories for Smart Drugs
A team of researchers has turned ordinary yeast into tiny, glowing drug factories, creating and testing billions of peptide-based compounds in record time. This green-tech breakthrough could fast-track safer, more precise medicines and reshape the future of pharma.

Alternative Medicine
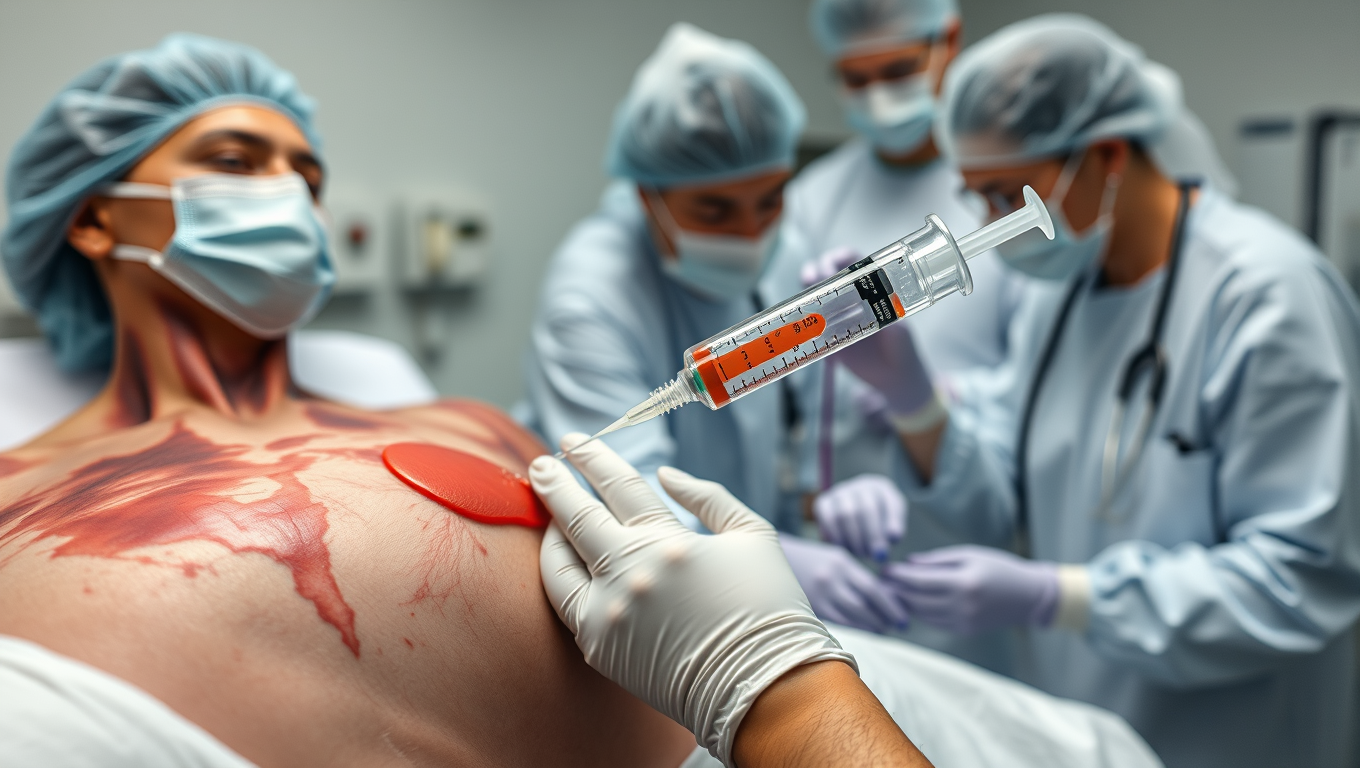
“Skin in a Syringe”: Breakthrough Technology Heals Burns without Scars
Scientists in Sweden have developed a groundbreaking “skin in a syringe” — a gel packed with live cells that can be applied directly to wounds or even 3D-printed into skin grafts. Designed to help the body build functional dermis rather than scar tissue, the innovation combines fibroblast cells on gelatin beads with a hyaluronic acid gel, held together using click chemistry. In a parallel advance, the team also created elastic hydrogel threads that can form tiny, fluid-carrying channels, paving the way for artificial tissues and organoid development.
Alternative Medicine
A 30-Minute Workout That Could Help Slash Cancer Cell Growth
A vigorous workout can spark anti-cancer proteins, cut cancer cell growth, and help survivors fight recurrence by reducing inflammation and improving body composition.
Alternative Medicine
Breaking Barriers in Diabetic Wound Healing: A Revolutionary “Smart” Gel Accelerates Blood Flow and Restores Tissue Repair
A new gel-based treatment could change the way diabetic wounds heal. By combining tiny healing messengers called vesicles with a special hydrogel, scientists have created a dressing that restores blood flow and helps wounds close much faster. In tests, the treatment healed diabetic wounds far quicker than normal, while also encouraging the growth of new blood vessels. Researchers believe this innovation could one day help millions of people with slow-healing wounds caused by diabetes and possibly other conditions.
-

 Detectors10 months ago
Detectors10 months agoA New Horizon for Vision: How Gold Nanoparticles May Restore People’s Sight
-

 Earth & Climate12 months ago
Earth & Climate12 months agoRetiring Abroad Can Be Lonely Business
-

 Cancer11 months ago
Cancer11 months agoRevolutionizing Quantum Communication: Direct Connections Between Multiple Processors
-

 Albert Einstein12 months ago
Albert Einstein12 months agoHarnessing Water Waves: A Breakthrough in Controlling Floating Objects
-

 Chemistry11 months ago
Chemistry11 months ago“Unveiling Hidden Patterns: A New Twist on Interference Phenomena”
-

 Earth & Climate11 months ago
Earth & Climate11 months agoHousehold Electricity Three Times More Expensive Than Upcoming ‘Eco-Friendly’ Aviation E-Fuels, Study Reveals
-

 Diseases and Conditions12 months ago
Diseases and Conditions12 months agoReducing Falls Among Elderly Women with Polypharmacy through Exercise Intervention
-

 Agriculture and Food11 months ago
Agriculture and Food11 months ago“A Sustainable Solution: Researchers Create Hybrid Cheese with 25% Pea Protein”