While we try to keep things accurate, this content is part of an ongoing experiment and may not always be reliable.
Please double-check important details — we’re not responsible for how the information is used.
COVID and SARS
COVID Vaccines Save 2.5 Million Lives Globally – One Death Averted per 5,400 Shots
Between 2020 and 2024, COVID-19 vaccines saved 2.5 million lives globally, preventing one death for every 5,400 doses. A groundbreaking worldwide study led by researchers from Università Cattolica and Stanford University reveals that most lives were saved before individuals were exposed to the virus, particularly during the Omicron period and among those aged 60+. The researchers also calculated 14.8 million years of life saved, with the elderly gaining the majority of these benefits.

Cold and Flu
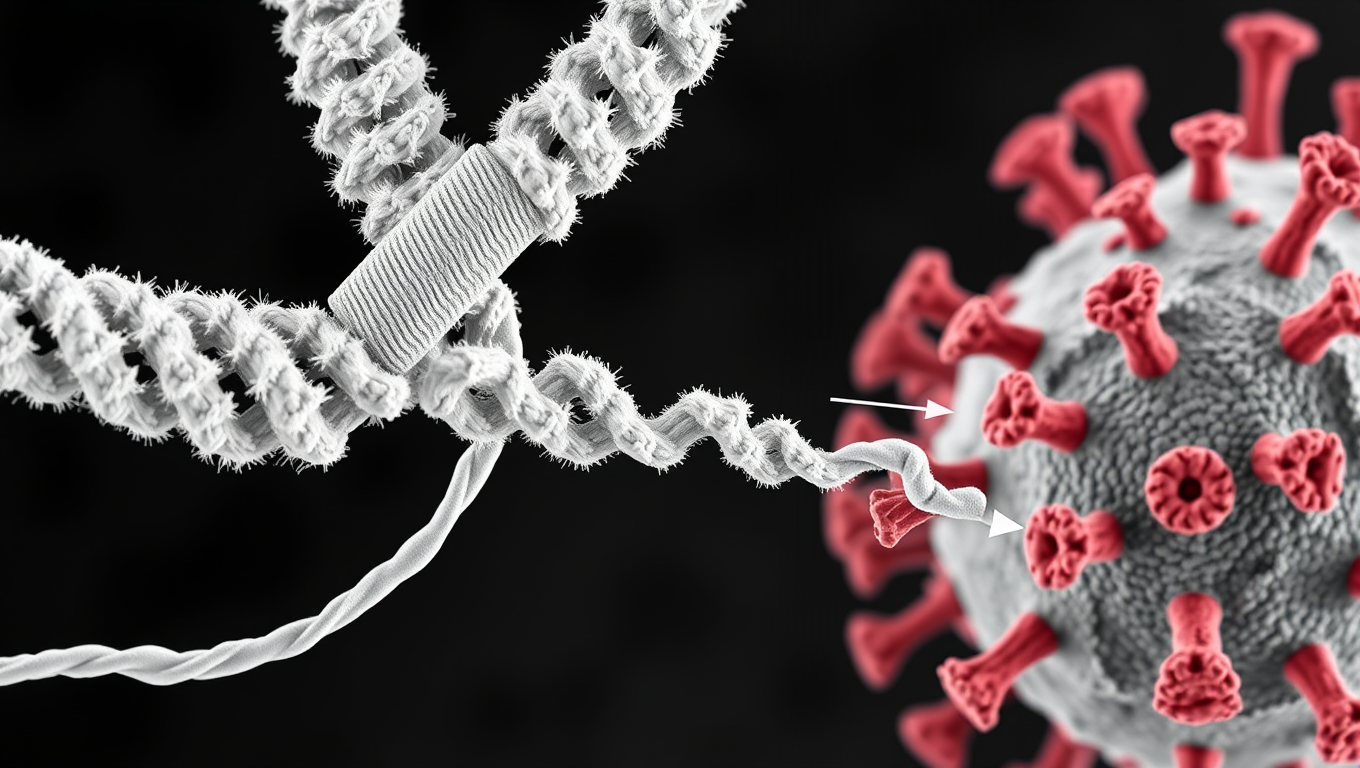
Scientists Discover Llama Antibodies That Shut Down COVID-19 and Its Future Variants
Powerful llama-derived antibodies could be the key to stopping not just current SARS viruses, but future ones too. Scientists have discovered a unique class of nanobodies that clamp the coronavirus spike protein shut at a highly conserved region, rendering it unable to infect cells. Unlike existing therapies that target mutating regions, this approach strikes at the virus s core machinery, giving it little room to evolve. Even when pushed to mutate, the virus faltered, making this a high-potential strategy for broad, lasting protection.
Biochemistry
A Game-Changing mRNA Vaccine that’s More Effective and Less Costly to Develop
A new type of mRNA vaccine is more scalable and adaptable to continuously evolving viruses such as SARS-CoV-2 and H5N1, according to a new study.
Bacteria
Engineered Bacteria Deliver Antiviral Therapies and Vaccines with Unprecedented Ease
New research demonstrates how specially engineered bacteria taken orally can operate as a delivery system for vaccines and antiviral therapies.
-

 Detectors10 months ago
Detectors10 months agoA New Horizon for Vision: How Gold Nanoparticles May Restore People’s Sight
-

 Earth & Climate11 months ago
Earth & Climate11 months agoRetiring Abroad Can Be Lonely Business
-

 Cancer11 months ago
Cancer11 months agoRevolutionizing Quantum Communication: Direct Connections Between Multiple Processors
-

 Albert Einstein11 months ago
Albert Einstein11 months agoHarnessing Water Waves: A Breakthrough in Controlling Floating Objects
-

 Chemistry10 months ago
Chemistry10 months ago“Unveiling Hidden Patterns: A New Twist on Interference Phenomena”
-

 Earth & Climate10 months ago
Earth & Climate10 months agoHousehold Electricity Three Times More Expensive Than Upcoming ‘Eco-Friendly’ Aviation E-Fuels, Study Reveals
-

 Agriculture and Food11 months ago
Agriculture and Food11 months ago“A Sustainable Solution: Researchers Create Hybrid Cheese with 25% Pea Protein”
-

 Diseases and Conditions11 months ago
Diseases and Conditions11 months agoReducing Falls Among Elderly Women with Polypharmacy through Exercise Intervention