While we try to keep things accurate, this content is part of an ongoing experiment and may not always be reliable.
Please double-check important details — we’re not responsible for how the information is used.
Dementia
Decoding Cell Development: Unveiling the Formation of the Brain and Inner Ear
Researchers have developed a method that shows how the nervous system and sensory organs are formed in an embryo. By labeling stem cells with a genetic ‘barcode’, they have been able to follow the cells’ developmental journey and discover how the inner ear is formed in mice. The discovery could provide important insights for future treatment of hearing loss.
Alzheimer's
Uncovering the Hidden Culprits Behind Alzheimer’s Disease
A surprising new study has uncovered over 200 misfolded proteins in the brains of aging rats with cognitive decline, beyond the infamous amyloid and tau plaques long blamed for Alzheimer’s. These shape-shifting proteins don’t clump into visible plaques, making them harder to detect but potentially just as harmful. Scientists believe these “stealth” molecules could evade the brain’s cleanup systems and quietly impair memory and brain function. The discovery opens a new frontier in understanding dementia and could lead to entirely new targets for treatment and prevention.
Alzheimer's
Uncovering the Hidden Defenses Against Alzheimer’s Disease: A Breakthrough Study on Brain Resilience
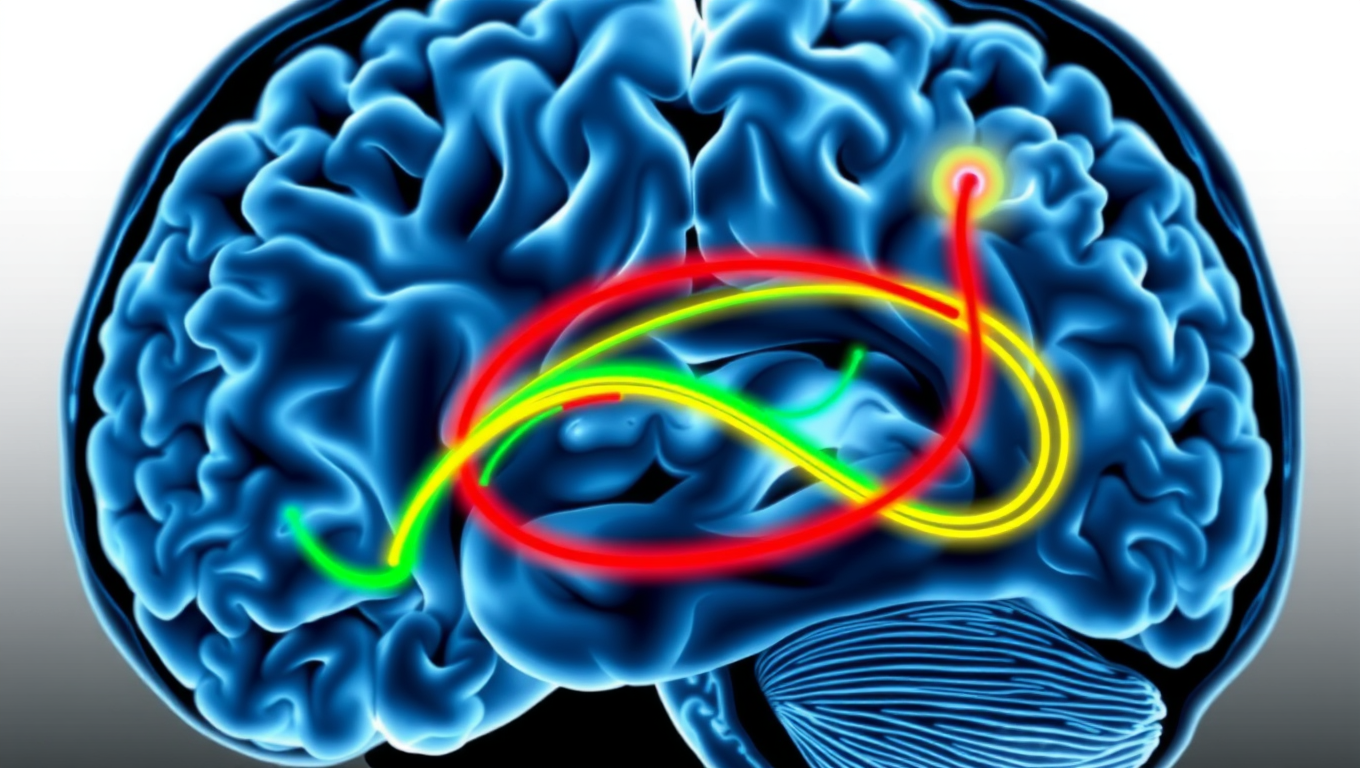
Scientists at UCSF combined advanced brain-network modeling, genetics, and imaging to reveal how tau protein travels through neural highways and how certain genes either accelerate its toxic journey or shield brain regions from damage. Their extended Network Diffusion Model pinpoints four gene categories that govern vulnerability or resilience, reshaping our view of Alzheimer’s progression and spotlighting fresh therapeutic targets.
Dementia
“Decoding Alzheimer’s: 4 Hidden Patterns That Reveal a Risky Path”
UCLA scientists mined millions of electronic health records and uncovered four distinct “roadways” that funnel people toward Alzheimer’s—ranging from mental-health struggles to vascular troubles. Following these breadcrumb trails proved far better at predicting who will develop dementia than single risk factors. The findings hint that spotting—and halting—specific sequences early could rewrite how we prevent the disease.
-

 Detectors3 months ago
Detectors3 months agoA New Horizon for Vision: How Gold Nanoparticles May Restore People’s Sight
-

 Earth & Climate4 months ago
Earth & Climate4 months agoRetiring Abroad Can Be Lonely Business
-

 Cancer4 months ago
Cancer4 months agoRevolutionizing Quantum Communication: Direct Connections Between Multiple Processors
-

 Agriculture and Food4 months ago
Agriculture and Food4 months ago“A Sustainable Solution: Researchers Create Hybrid Cheese with 25% Pea Protein”
-

 Diseases and Conditions4 months ago
Diseases and Conditions4 months agoReducing Falls Among Elderly Women with Polypharmacy through Exercise Intervention
-

 Albert Einstein4 months ago
Albert Einstein4 months agoHarnessing Water Waves: A Breakthrough in Controlling Floating Objects
-

 Chemistry4 months ago
Chemistry4 months ago“Unveiling Hidden Patterns: A New Twist on Interference Phenomena”
-

 Earth & Climate4 months ago
Earth & Climate4 months agoHousehold Electricity Three Times More Expensive Than Upcoming ‘Eco-Friendly’ Aviation E-Fuels, Study Reveals