While we try to keep things accurate, this content is part of an ongoing experiment and may not always be reliable.
Please double-check important details — we’re not responsible for how the information is used.
Alzheimer's
Scientists Uncover Secrets of Protein Blobs That Morph from Liquid to Solid in Human Cells
Scientists examined microscopic blobs of protein found in human cells has discovered that some morph from an almost honey-like substance to a hard candy-like solid. These mysterious droplets, known as biomolecular condensates, solidify when they carry a high proportion of the protein alpha-synuclein, the scientists reported. Clumps of alpha-synuclein are commonly found in the brain cells of people with Parkinson’s disease, a neurodegenerative brain disorder.
Alzheimer's
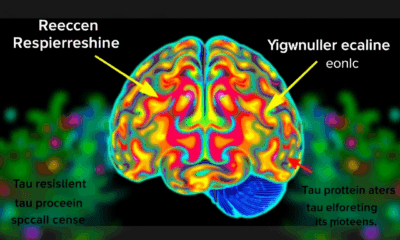
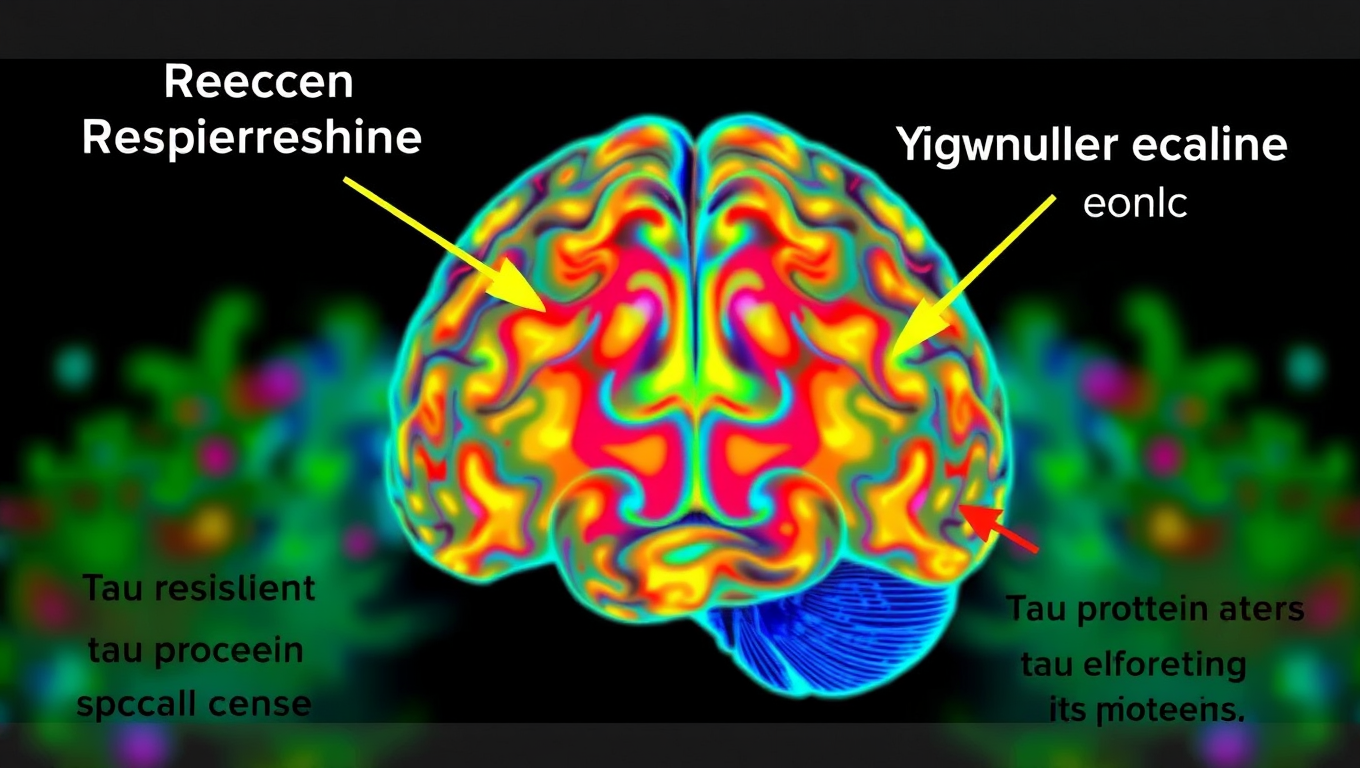
Uncovering the Hidden Defenses Against Alzheimer’s Disease: A Breakthrough Study on Brain Resilience
Scientists at UCSF combined advanced brain-network modeling, genetics, and imaging to reveal how tau protein travels through neural highways and how certain genes either accelerate its toxic journey or shield brain regions from damage. Their extended Network Diffusion Model pinpoints four gene categories that govern vulnerability or resilience, reshaping our view of Alzheimer’s progression and spotlighting fresh therapeutic targets.
Alzheimer's
Unlocking the Brain’s Sugar Code: Scientists Discover a New Player in the Battle Against Alzheimer’s
Scientists have uncovered a surprising sugar-related mechanism inside brain cells that could transform how we fight Alzheimer’s and other dementias. It turns out neurons don’t just store sugar for fuel—they reroute it to power antioxidant defenses, but only if an enzyme called GlyP is active. When this sugar-clearing system is blocked, toxic tau protein builds up and accelerates brain degeneration.
Alzheimer's
Groundbreaking Study Suggests Link Between Semaglutide and Lower Dementia Risk in Type 2 Diabetes Patients
A blockbuster diabetes and weight-loss drug might be doing more than controlling blood sugar—it could also be protecting the brain. Researchers at Case Western Reserve University found that people with type 2 diabetes who took semaglutide (the active ingredient in Ozempic and Wegovy) had a significantly lower risk of developing dementia. The benefit was especially strong in women and older adults.
-

 Detectors3 months ago
Detectors3 months agoA New Horizon for Vision: How Gold Nanoparticles May Restore People’s Sight
-

 Earth & Climate4 months ago
Earth & Climate4 months agoRetiring Abroad Can Be Lonely Business
-

 Cancer4 months ago
Cancer4 months agoRevolutionizing Quantum Communication: Direct Connections Between Multiple Processors
-

 Agriculture and Food4 months ago
Agriculture and Food4 months ago“A Sustainable Solution: Researchers Create Hybrid Cheese with 25% Pea Protein”
-

 Diseases and Conditions4 months ago
Diseases and Conditions4 months agoReducing Falls Among Elderly Women with Polypharmacy through Exercise Intervention
-

 Albert Einstein4 months ago
Albert Einstein4 months agoHarnessing Water Waves: A Breakthrough in Controlling Floating Objects
-

 Chemistry3 months ago
Chemistry3 months ago“Unveiling Hidden Patterns: A New Twist on Interference Phenomena”
-

 Earth & Climate4 months ago
Earth & Climate4 months agoHousehold Electricity Three Times More Expensive Than Upcoming ‘Eco-Friendly’ Aviation E-Fuels, Study Reveals