While we try to keep things accurate, this content is part of an ongoing experiment and may not always be reliable.
Please double-check important details — we’re not responsible for how the information is used.
Brain Injury
The Long-term Effects of Obesity on Brain and Cognitive Health: A Dynamic Relationship Revealed
With the global prevalence of obesity on the rise, it is crucial to explore the neural mechanisms linked to obesity and its influence on brain and cognitive health. However, the impact of obesity on the brain is complex and multilevel.
Alzheimer's
Groundbreaking Study Suggests Link Between Semaglutide and Lower Dementia Risk in Type 2 Diabetes Patients
A blockbuster diabetes and weight-loss drug might be doing more than controlling blood sugar—it could also be protecting the brain. Researchers at Case Western Reserve University found that people with type 2 diabetes who took semaglutide (the active ingredient in Ozempic and Wegovy) had a significantly lower risk of developing dementia. The benefit was especially strong in women and older adults.
Amyotrophic Lateral Sclerosis
“Reviving Memories: Gene Therapy Shows Promise in Reversing Alzheimer’s Disease in Mice”
UC San Diego scientists have created a gene therapy that goes beyond masking Alzheimer’s symptoms—it may actually restore brain function. In mice, the treatment protected memory and altered diseased brain cells to behave more like healthy ones.
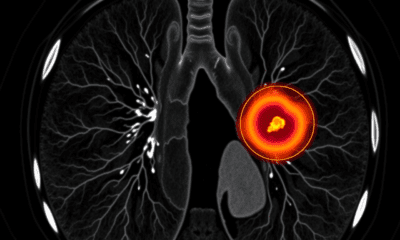
Brain Injury
The Hidden Glitch Behind Hunger: Scientists Uncover the Brain Cells Responsible for Meal Memories
A team of scientists has identified specialized neurons in the brain that store “meal memories” detailed recollections of when and what we eat. These engrams, found in the ventral hippocampus, help regulate eating behavior by communicating with hunger-related areas of the brain. When these memory traces are impaired due to distraction, brain injury, or memory disorders individuals are more likely to overeat because they can’t recall recent meals. The research not only uncovers a critical neural mechanism but also suggests new strategies for treating obesity by enhancing memory around food consumption.
-

 Detectors3 months ago
Detectors3 months agoA New Horizon for Vision: How Gold Nanoparticles May Restore People’s Sight
-

 Earth & Climate4 months ago
Earth & Climate4 months agoRetiring Abroad Can Be Lonely Business
-

 Cancer3 months ago
Cancer3 months agoRevolutionizing Quantum Communication: Direct Connections Between Multiple Processors
-

 Agriculture and Food3 months ago
Agriculture and Food3 months ago“A Sustainable Solution: Researchers Create Hybrid Cheese with 25% Pea Protein”
-

 Diseases and Conditions4 months ago
Diseases and Conditions4 months agoReducing Falls Among Elderly Women with Polypharmacy through Exercise Intervention
-

 Earth & Climate3 months ago
Earth & Climate3 months agoHousehold Electricity Three Times More Expensive Than Upcoming ‘Eco-Friendly’ Aviation E-Fuels, Study Reveals
-

 Chemistry3 months ago
Chemistry3 months ago“Unveiling Hidden Patterns: A New Twist on Interference Phenomena”
-

 Albert Einstein4 months ago
Albert Einstein4 months agoHarnessing Water Waves: A Breakthrough in Controlling Floating Objects