While we try to keep things accurate, this content is part of an ongoing experiment and may not always be reliable.
Please double-check important details — we’re not responsible for how the information is used.
Brain Tumor
A New Path Forward: Unlocking Brain Cell Activity with Potassium Channels to Treat Depression
A mechanism involving potassium channels in the brain that control brain cell activity could provide a new and fundamentally different way of treating depression symptoms in adults with major depressive disorder.

Brain Tumor
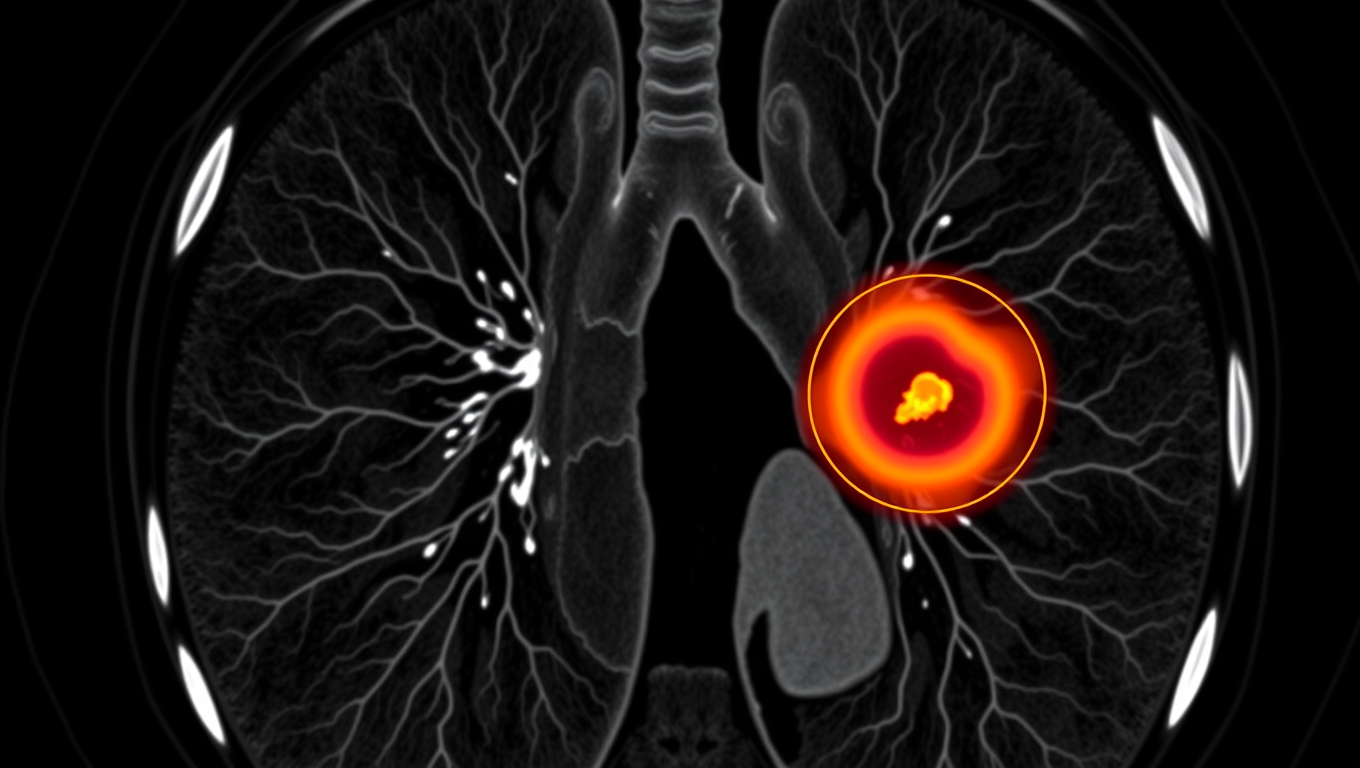
AI Tool Tracks Lung Tumors as You Breathe, Potentially Saving Lives
An AI system called iSeg is reshaping radiation oncology by automatically outlining lung tumors in 3D as they shift with each breath. Trained on scans from nine hospitals, the tool matched expert clinicians, flagged cancer zones some missed, and could speed up treatment planning while reducing deadly oversights.
Brain Injury
The Hidden Glitch Behind Hunger: Scientists Uncover the Brain Cells Responsible for Meal Memories
A team of scientists has identified specialized neurons in the brain that store “meal memories” detailed recollections of when and what we eat. These engrams, found in the ventral hippocampus, help regulate eating behavior by communicating with hunger-related areas of the brain. When these memory traces are impaired due to distraction, brain injury, or memory disorders individuals are more likely to overeat because they can’t recall recent meals. The research not only uncovers a critical neural mechanism but also suggests new strategies for treating obesity by enhancing memory around food consumption.
Brain Tumor
Uncovering Nature’s Secret: Ginger Compound Shows Promise in Targeting Cancer Cells’ Metabolism
Scientists in Japan have discovered that a natural compound found in a type of ginger called kencur can throw cancer cells into disarray by disrupting how they generate energy. While healthy cells use oxygen to make energy efficiently, cancer cells often rely on a backup method. This ginger-derived molecule doesn t attack that method directly it shuts down the cells’ fat-making machinery instead, which surprisingly causes the cells to ramp up their backup system even more. The finding opens new doors in the fight against cancer, showing how natural substances might help target cancer s hidden energy tricks.
-

 Detectors10 months ago
Detectors10 months agoA New Horizon for Vision: How Gold Nanoparticles May Restore People’s Sight
-

 Earth & Climate11 months ago
Earth & Climate11 months agoRetiring Abroad Can Be Lonely Business
-

 Cancer11 months ago
Cancer11 months agoRevolutionizing Quantum Communication: Direct Connections Between Multiple Processors
-

 Albert Einstein11 months ago
Albert Einstein11 months agoHarnessing Water Waves: A Breakthrough in Controlling Floating Objects
-

 Chemistry10 months ago
Chemistry10 months ago“Unveiling Hidden Patterns: A New Twist on Interference Phenomena”
-

 Earth & Climate10 months ago
Earth & Climate10 months agoHousehold Electricity Three Times More Expensive Than Upcoming ‘Eco-Friendly’ Aviation E-Fuels, Study Reveals
-

 Agriculture and Food11 months ago
Agriculture and Food11 months ago“A Sustainable Solution: Researchers Create Hybrid Cheese with 25% Pea Protein”
-

 Diseases and Conditions11 months ago
Diseases and Conditions11 months agoReducing Falls Among Elderly Women with Polypharmacy through Exercise Intervention